
Whereas in addition to polymerization, the water molecule is not used up, it’s just not released as a by-product. Monomer that combine to form polymers include: Glucose molecules combine to form cellulose and starch. A polymer can either occur naturally e.g starch, cellulose etc. One way to think about it is that in condensation polymerization, the water molecule is “consumed” in the process of forming the larger molecule. In a polymer, two or more monomers are joined together by chemical bonds. In organic macromolecules (there may be other instances, but this is the best example), they are formed through a dehydration synthesis reaction (also called a condensation reaction). In addition to polymerization, two or more monomers join together to form a bigger molecule, and no water is released as a by-product. Explanation: Multiple monomers together form a molecule called a polymer. In condensation polymerization, two molecules join together to form a larger molecule, and water is released as a by-product. Small unit that can join together with other small units to form a. Also two oligomers can join together and form a polymer. When the monomers combine they make -OH bonds leaving behind single H’s! When reading Condensation Polymoners you have (-OH) bonded (+H). Two monomers join together to form a polymer. In addition, Polymerizations are easier to “track”. This is the type of reaction that occurs when polyethylene is made from ethylene gas.Ĭondensation Polymerizations are the opposite due to its mechanism. True T/F Waters ability to form solutions is due to its polarity.

False T/F A hydrogen bond is stronger than a covalent bond. False T/F Covalent bonds give water a low heat capacity. False T/F Hydrogen bonds are an example of adhesion. In an addition polymerization reaction, monomers are added together to form a polymer chain. polymer macromolecule formed when monomers join together True T/F Water is a polar molecule. This is the type of reaction that occurs when nylon is made from caprolactam and hexamethylenediamine. In a condensation polymerization reaction, two molecules join together to form a larger molecule, and water is released as a by-product. Difference between Addition Polymerization and Condensation Polymerization Reaction: Examples include ABS (Acrylonitrile Butadiene Styrene) and PPS (Polyphenylsulfide). Thus it is generally used in materials science and engineering when the properties in the 2D plane of material are undesired for application in architecture or engineering. In addition, polymers form 3D structures by themselves, they do not need another agent to provide this. Monomers may be the same or they may be different. This process is used to make plastics, like polyethylene, from ethylene gas.ĪDDITION POLYMERIZATION is an atom-by-atom reaction, where two unactivated monomers react to form a polymer. The small molecules are all added together and there is no loss of any of the individual molecules. In addition to polymerization, the small molecules join together to form one big molecule.
#WHEN TWO OR MORE JOIN TOGETHER A POLYMER FORMS FREE#
With additional polymerization, the smaller molecules are typically identical monomers.ĬONDENSATION POLYMERIZATION is where one molecule reacts with itself by adding four gaseous chemical groups called free radicals What is Addition Polymerization? In condensation polymerization is a polymer that involves a condensation reaction, generally the smaller molecules are usually water and either a functional group or a monomer unit. In a condensation reaction, one molecule splits into two smaller molecules. In an additional reaction, two small molecules join together to form one larger molecule. In general, there are two types of polymerization reactions: addition and condensation. The two products are called monomers and the resulting polymer belongs to one of three types: addition polymerization, condensation polymerization, or ring-opening polymerization.
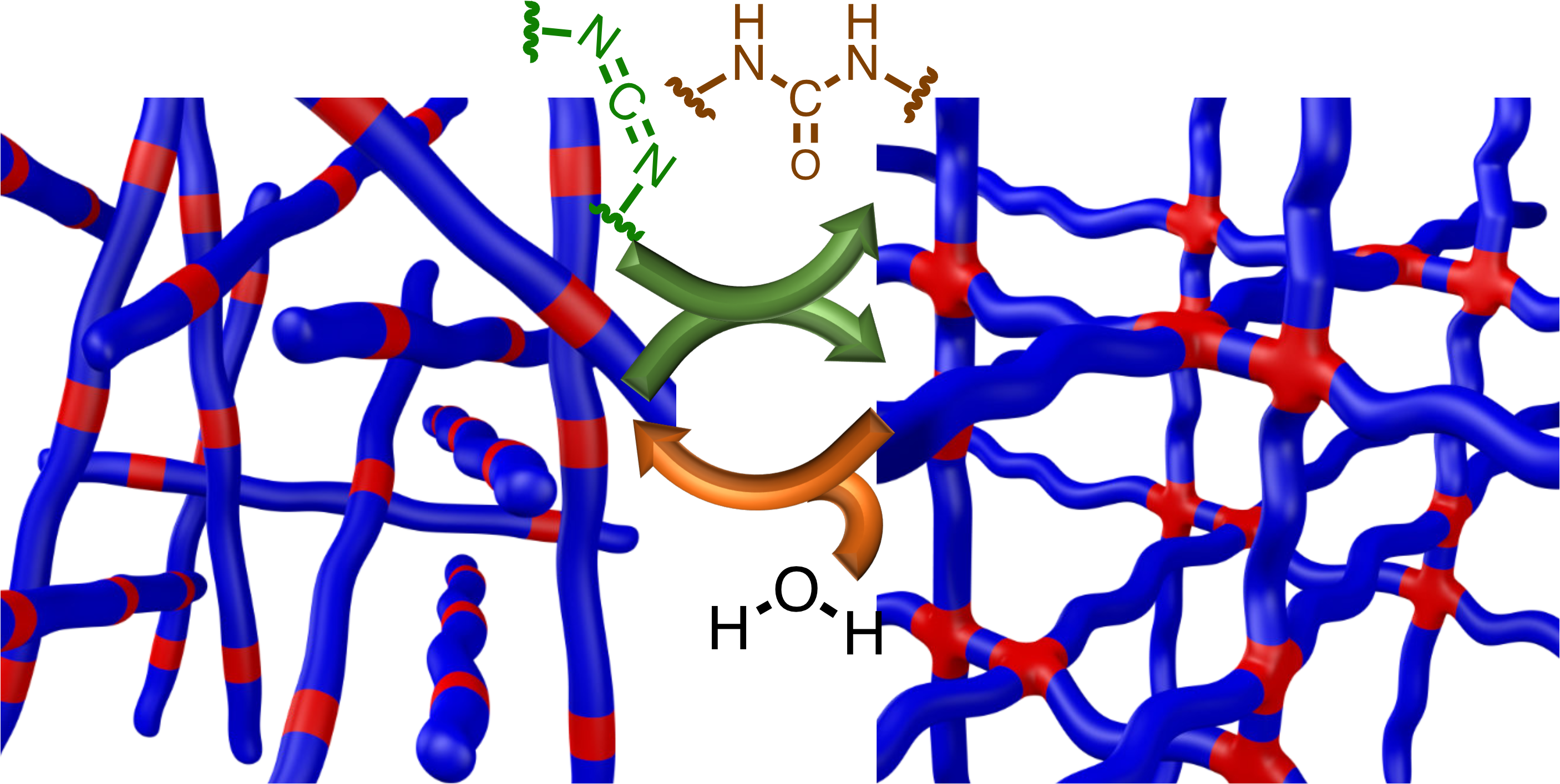
This process is used to create an entirely new product by linking together molecules of two different products. Polymerization is a chemical process that yields chains of repetitive units called polymers. There are two types of polymerization: addition and condensation. Polymerization is a process where small molecules join together to form bigger molecules. Transcription and Translation in ProkaryotesĮxamples of monosaccharides include glucose, galactose, and fructose.Difference between Addition Polymerization and Condensation Polymerization? What is Polymerization?.Changes in Signal Transduction Pathways.


 0 kommentar(er)
0 kommentar(er)
